KUTTAM CELLULAR AND MOLECULAR IMAGING CORE (KUTTAM-CMIC) INFORMATION GUIDE
Dear Researchers,
In accordance with the latest evaluations and needs, the general operation principles are updated as follows.
GENERAL OPERATION PRINCIPLES
• Users are allowed to access and use the microscope systems only after completing theoretical and practical training sessions.
• Institutional users can make reservations through the “bookkit” (https://www.clustermarket.com/) calendar if their user information is defined. To define new user, those who do not have an account should contact Sema Balı (sbali@ku.edu.tr) for KUH and Büşra Harmanda (bharmanda@ku.edu.tr) for RF.
• It is not possible to use the systems without a reservation.
• All groups will be charged for using the microscopes starting 1st April 2023. Please set a budget for imaging on your project proposals.
• A reservation can be made through the system only for 3 following weeks and at least 4 days in advance. We will not accept late reservation requests anymore, with no exception. In case of urgent need, your PI should reach out to us.
• Note that reservations must be confirmed by the responsible personnel.
• In case of late cancellation (two days before the session) you will be charged 50% of a regular price. It is necessary to be at the center on time. Appointments should not exceed the designated hours. In case of non-compliance with the specified hours the researcher will be warned, and the responsible PI will be informed. Restricted access may be applied in case of repetition.
• You should contact the CMIC staff by email for your questions/requests. Only for very urgent situations you should try to reach the CMIC staff by phone/WhatsApp.
• Training sessions are limited and are planned according to our schedule. There is no ‘’urgent training’’ session and you should wait in the queue, even if you have project deadline, thesis defense, etc.
• The process computers and the provided hard disks will be formatted regularly, please remove your data immediately after your sections, otherwise it will be deleted without notice.
Regulations specific to Rumelifeneri Campus (RF):
For the Confocal microscope, a reservation section is limited to 4 hours and should be in the following time slots:
09:00 – 13:00 (Weekdays only)
13:00 – 17.00 (Weekdays only)
• A user can reserve only one section in a day.
• Each research group can reserve maximum two sections in a week. If a group has more than two reservations in a week, we have the authority to cancel your reservations in case of high demand (even if it is already approved). So, try to plan your experiments accordingly, to prevent facing the risk of cancellation.
• CMIC-RF continues to operate after working hours, but this is limited to Senior Authorized Users having enough experience and expertise. The Time slots for expert users are:
Weekdays: 17:00 – 21:00 21:00 – next day 09:00
Weekends: No time sectioning.
• An authorized expert user can reserve maximum 24 hours in a week (including weekends). We have the authority to cancel more than 24 hours reservations in case of high demand (even it is already approved). So, try to plan your experiments accordingly, to prevent facing the risk of cancellation. For more than 24 hours, prearrangement should be done with Büşra Harmanda (bharmanda@ku.edu.tr).
Regulations specific to Koç University Hospital (KUH)
For the Confocal microscope, a reservation section is limited to 4 hours and should be in the following time slots:
09:00 – 13:00 (Weekdays only)
13:00 – 17.00 (Weekdays only)
• A user can reserve only one section in a day.
• Each research group can reserve maximum Three sections in a week. If a group has more than two reservations in a week, we have the authority to cancel your reservations in case of high demand (even if it is already approved). So, try to plan your experiments accordingly, to prevent facing the risk of cancellation.
• CMIC-RF continues to operate after working hours, but this is limited to Senior Authorized Users having enough
experience and expertise. The Time slots for expert users are:
Weekdays: 17:00 – 22:00 22:00 – next day 09:00
Weekends: No time sectioning.
• An authorized expert user can reserve maximum 24 hours in a week (including weekends). We have the authority to cancel more than 24 hours reservations in case of high demand (even it is already approved). So, try to plan your experiments accordingly, to prevent facing the risk of cancellation. For more than 24 hours, prearrangement should be done with Sema Balı (sbali@ku.edu.tr).
PAYMENT INFORMATION
• Institutional (KU) and Non-institutional (non-KU) pricing takes place as indicated in the table below.
• For payment details, KUTTAM@ku.edu.tr can be contacted.
• Please set a budget for imaging on Project proposals.
| Microscope System | Institutional (KU) | Non-Institutional |
| Leica DMI8/SP8 Confocal System | 150 TL + tax /hour | 660TL + tax /hour |
| Leica Dmi8/SP8 DLS/STED ve | 225 TL + tax /hour | 990 TL + tax /hour |
| Leica DMI8/SP8 DLS/STED and Leica DM6/SP8 MP Systems | ||
| Leica DMI8 and Leica DMI8/SP8 live cell imaging systems/ Zeiss Live cell/ Zeiss FL | 375 TL + tax /12 hour | 1050 TL + tax /12 hour |
| 750 TL + tax /24 hour | 2100 TL + tax /24 hour | |
| 1500 TL + tax /72 hour | 5400 TL + tax /72 hour | |
| Multiphoton Mikroskop | 500 TL + tax/hour | 1000 TL + tax/hour |
RECOVERY, PROCESSING AND STORAGE OF DATA
• Images obtained during use are transferred to the data processing computer in the center with the external disk provided by KUTTAM-CMIC. Users can transfer data from this computer to their own memory.
• A pc compatible link with many functions of the LASX application suite can be transmitted via e-mail to the requesting users for installation on their own computers.
• Users can use the data processing computer located in the center for the operations they cannot perform with the LASX program installed on their own computers. This computer also needs to be reserved before use like systems.
• Users’ data are deleted after they have been stored in the central computer for a maximum of 3 (three) months. For this reason, it is necessary for users to take their data before this time.
CONTACT
Location: Koç University, Research Center for Translational Medicine
Center of Koç Hospital: Koç University Hospital, KUTTAM 4th Floor, Topkapı
Center of RF: Koç University Rumelifeneri Campus, SNA B203A, Sarıyer
Responsible Faculty:
Serçin Karahüseyinoğlu, MD.
Tel: +90 (212) 338-1161
E-mail: skarahuseyinoglu@ku.edu.tr
Alper Kiraz, Prof. Dr.
Tel: +90 (212) 338-1701
E-mail: akiraz@ku.edu.tr
Responsible Personnel:
KUH: Sema Balı
E-mail: sbali@ku.edu.tr
RF: Büşra Harmanda, PhD
E-mail: bharmanda@ku.edu.tr
DLS




MP
STED
E-Mail: skarahuseyinoglu@ku.edu.tr
E-Mail: akiraz@ku.edu.tr
Örnek kalınlığı mikroskopide önemli bir parametredir ve kalınlık arttıkça görüntü alınabilmesi için ışığın daha derine girmesi gereklidir. Multifoton eksitasyon mikroskobu özellikle kalın örneklerde (beyin dilimleri, embriyolar, organın tümü ve hatta bazı durumlarda hayvanın tümü) görüntülemeye olanak sağlamaktadır. Sistemde bulunan kızıl ötesi eksitasyon lazerleri dokulara derin penetrasyonu sağlamakta ve, hücreler ve hücre içindeki yapılar detaylı şekilde ortaya çıkarılmaktadır. Özellikle canlı örneklerde fluoresan görüntülemenin en önemli sınırlayıcı parametrelerinden biri olan foto-solmanın, kullanılan görüntüleme sistemi ile çok daha az izlenmesi önemli bir avantaj sağlamaktadır.
Canlı hücrelerin görüntülenmesi hücrenin fizyolojik işlevlerinin gerçek zamanlı olarak ortaya konulmasını sağlayan bir yöntemdir. Hücrenin işlevlerini yerine getirirken izlenebilmesi, ve in vitro hücresel tepkilerin görülmesi, hücre davranışının değişmesini sağlayacak yaklaşımların oluşturulmasında kritik rol oynar. Hücrelerin uzun süreli gözlemlerde dahi sağlıklı ve canlı kalmalarının sağlanabilmesi için bu sistem sıcaklık, pH, nem ve ışık kontrolünün sağlandığı inkübasyon ekipmanı ile donatılmıştır. Canlı görüntülemede önemli bir problem olan odak ayarının otomatik olarak sağlanması ise uzun süreleri inkübasyonu desteklemektedir. Sistem flüoresan görüntü sağlanabilmesine olanak sağlayarak hem transfekte canlı hücrelerin görüntülenmesine, hem de flüoresan konjuge boyalarla hazırlanmış örneklerin gözlenmesine olanak sağlamaktadır.
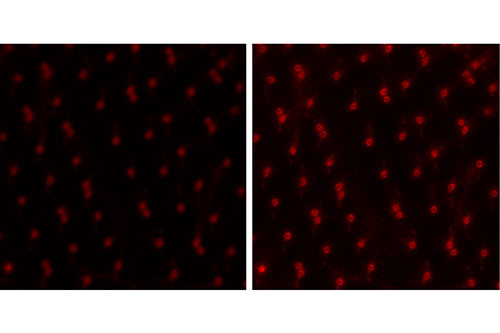
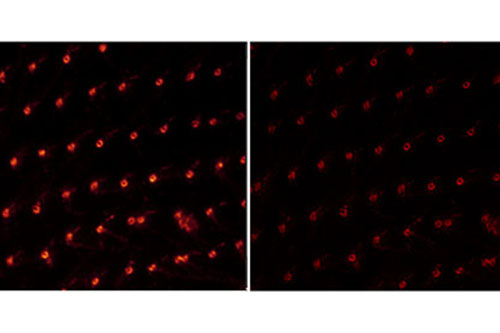
Mikroskopide son yıllarda yer alan en heyecan verici gelişmelerden bir tanesi hem lateral hem de aksiyel olarak nanometre skalasında çözünürlük sağlayan süperçözünürlük mikroskoplarıdır. KUTTAM’da bulunan süperçözünürlük mikroskobu STED (Stimulated Emission Depletion) teknolojisini kullanmakta ve 20-40 nanometreye kadar çözünürlük sağlanabilmektedir. Bu derece yüksek çözünürlük hücre içinde bulunan yapıların konvansiyonel kullanımdaki bir konfokal mikroskoptaki görüntülemeye göre çok daha detaylı şekilde ortaya çıkarılmasını sağlamaktadır. Ayrıca bu mikroskop sisteminde bulunan beyaz ışık lazeri (white light laser), yüksek çözünürlükte çok-renkli görüntüleme yapılmasına ve farklı yapıların keskin şekilde görülmesine olanak sağlamaktadır. Canlı görüntüleme de yapılabilen bu mikroskop sistemi özellikle ince örneklerde hücre işlevleri sırasında süper çözünürlükte görüntüleme yapılmasına olanak vermektedir.
Görüntüleme için ışık gereklidir ancak çok fazla ışık hücrelere zarar verebilir. DLS’ nin örneklerde oluşabilecek foto-hasar ve foto-solmayı azaltan son derece hassas bir teknik olması nedeniyle örnek canlılığı korunabilmektedir. Düşük ışık aydınlatmasının yüksek hızda görüntüleme ile birleştirildiği bu sistem özellikle doku ve organların oluşumu ve gelişimi sırasında izlenen hızlı ve periyodik işlevlerin uzun süreli üç-boyutlu görüntülemesi için kullanılmaktadır. Canlı örneklerin yanı sıra hazırlanmış örneklerin de üç-boyutlu olarak görüntülenmesine olanak sağlayan sistemle birlikte bulunan konfokal mikroskop ve gelişmiş dekonvolusyon sistemleri, örneklerde yüksek çözünürlükte hızlı görüntülemeye olanak sağlamaktadır.
